EX-99.1
Published on October 5, 2017

exploring new pathways to cancer therapy HedgePath Pharmaceuticals, Inc. 1 © 2017 HedgePath Pharmaceuticals, Inc. All rights reserved Exhibit 99.1

Cautionary Note on Forward-Looking Statements; No Offer of Securities This presentation includes or incorporates by reference statements that constitute “forward-looking statements” within the meaning of the U.S. federal securities laws. These statements relate to future events or to our future performance, and involve significant known and unknown risks, uncertainties and other factors that may cause our actual results, levels of activity, performance, or achievements to be materially different from any future results, levels of activity, performance or achievements expressed or implied in these forward-looking statements. These statements include, but are not limited to, information or assumptions about our clinical development programs, our expenses, capital and other expenditures, our financing needs and plans, our capital structure, and management’s plans, goals and objectives for future operations and growth. In some cases, you can identify forward-looking statements by the use of words such as “may,” “could,” “expect,” “intend,” “plan,” “seek,” “anticipate,” “believe,” “estimate,” “predict,” “continue,” “or the negative of these terms or other comparable terminology. You should not place undue reliance on forward-looking statement since they involve known and unknown risks, uncertainties and other factors which are, in some cases, beyond our control and which could cause actual performance or results to differ materially from those expressed in or suggested by forward-looking statements. Important factors that could cause such differences include, but are not limited to: (i) risks and uncertainties associated with our research and development activities, including our anticipated clinical trials; (ii) our dependence on Mayne Pharma for the supply of our product candidate and key intellectual property; (iii) our ability to raise capital when needed; (iv) the timing of and our ability to achieve U.S. or international regulatory approvals for our product candidates; (v) our dependence on others to conduct clinical of, and to manufacture and market, our product candidates; (vi) our ability to gain market acceptance for our product candidates; (vii) our ability to maintain or protect the validity of patents and other intellectual property; (viii) our ability to secure registration for our current and future patent applications; and (ix) our ability to attract and retain key personnel. Should one or more of these risks or uncertainties materialize, or should any of our assumptions prove incorrect, actual results may vary in material or even significant respects from those projected in these forward-looking statements. We do not undertake any obligation to update or revise any forward-looking statements, whether as a result of new information, future events or otherwise, except as may be required under applicable securities laws. This presentation does not constitute an offer or any securities for sale or solicitation of an offer to buy any securities, nor shall there be any sale of the securities in any state or jurisdiction in which such an offer, solicitation or sale would be unlawful prior to the registration or qualification under the securities laws of any such state or jurisdiction. 2 © 2017 HedgePath Pharmaceuticals, Inc. All rights reserved
3 © 2017 HedgePath Pharmaceuticals, Inc. All rights reserved HPPI OTCQX – Headquartered in Tampa, Florida
Hedgehog Pathway - Transmits signals for embryonic development - Regulates adult stem cells and tissue maintenance 4 © 2017 HedgePath Pharmaceuticals, Inc. All rights reserved Significant Molecular Pathway in Cancer - A Validated Cancer Target - Two FDA approved Hh Inhibitors - Toxicity is a major issue
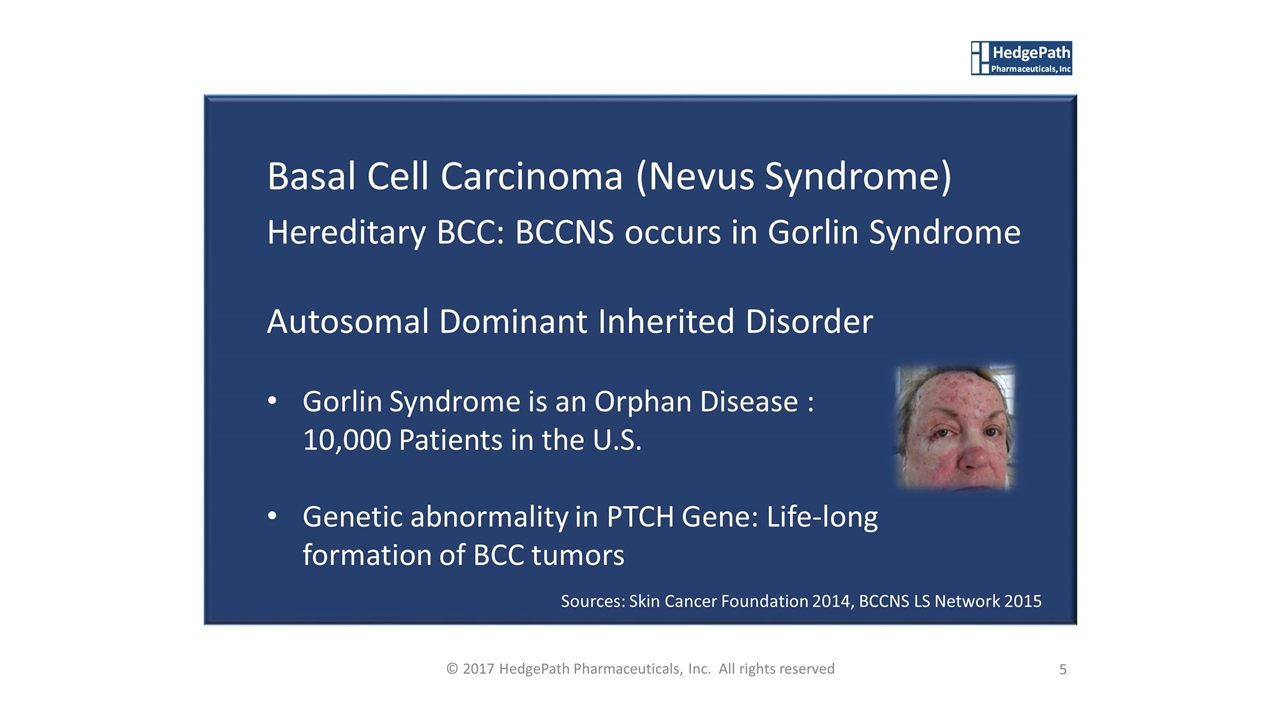
Basal Cell Carcinoma (Nevus Syndrome) Hereditary BCC: BCCNS occurs in Gorlin Syndrome Autosomal Dominant Inherited Disorder Gorlin Syndrome is an Orphan Disease : 10,000 Patients in the U.S. Genetic abnormality in PTCH Gene: Life-long formation of BCC tumors 5 © 2017 HedgePath Pharmaceuticals, Inc. All rights reserved Sources: Skin Cancer Foundation 2014, BCCNS LS Network 2015
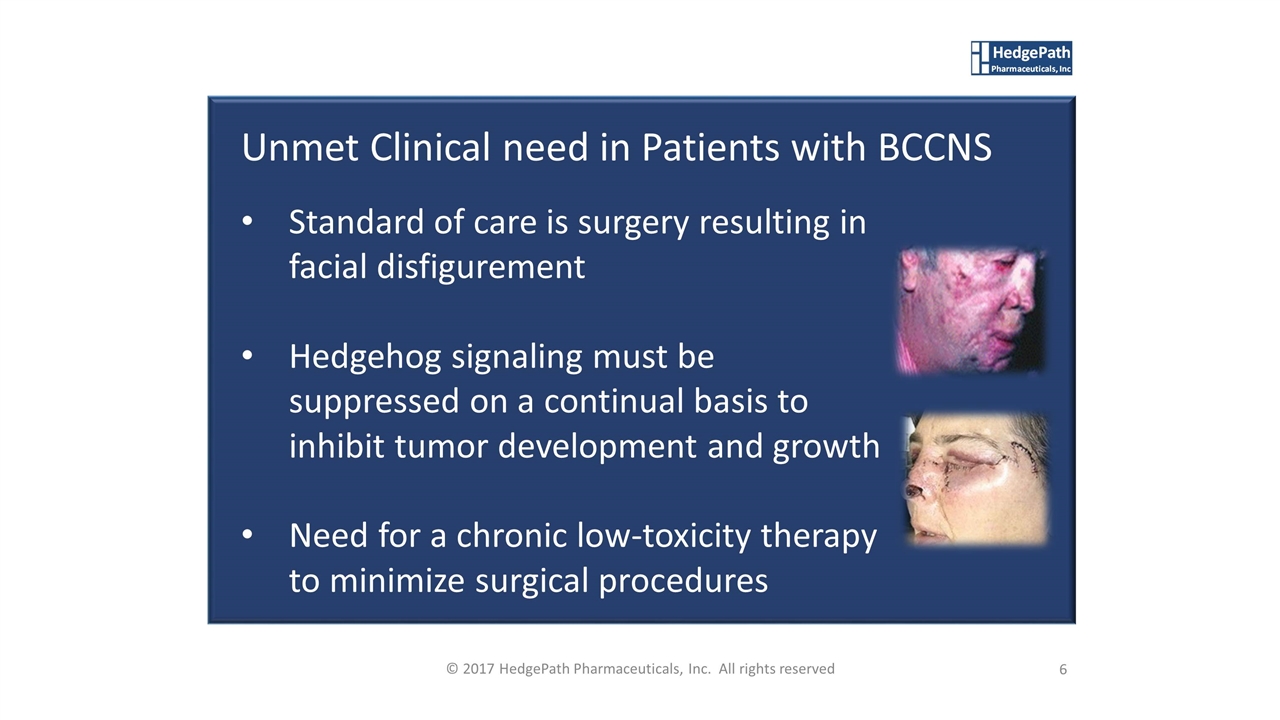
Unmet Clinical need in Patients with BCCNS Standard of care is surgery resulting in facial disfigurement Hedgehog signaling must be suppressed on a continual basis to inhibit tumor development and growth Need for a chronic low-toxicity therapy to minimize surgical procedures 6 © 2017 HedgePath Pharmaceuticals, Inc. All rights reserved
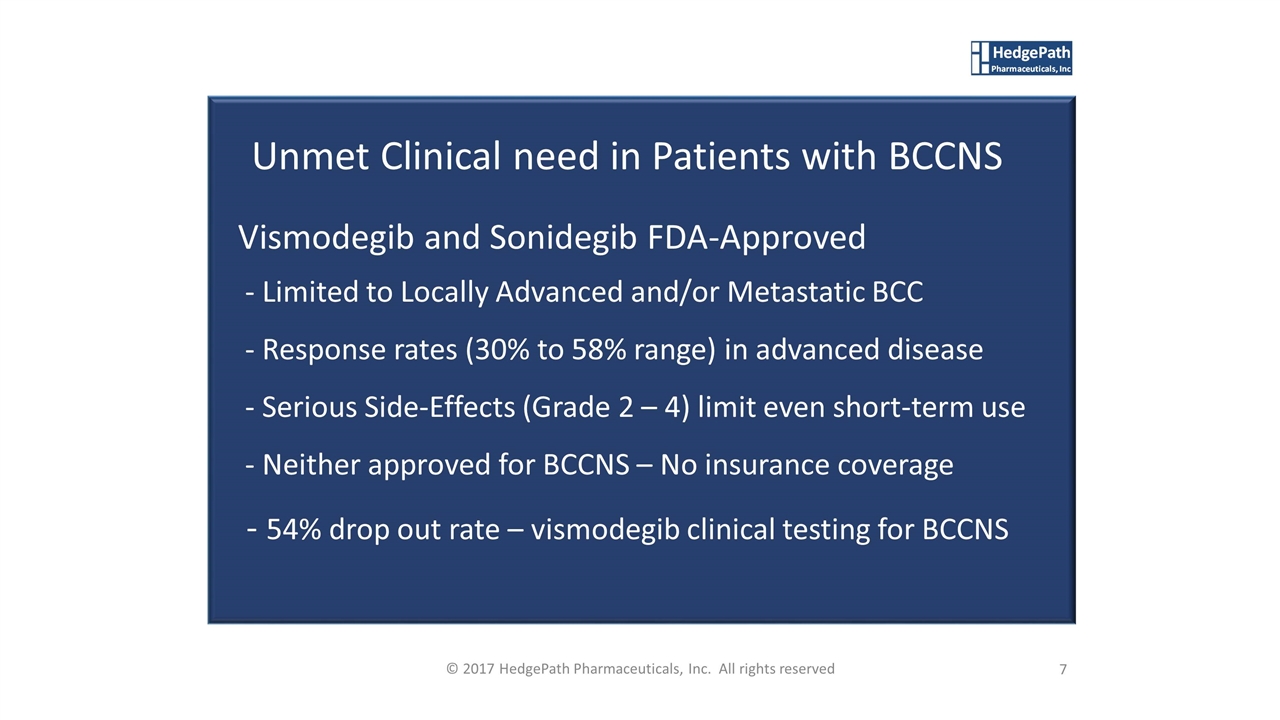
Unmet Clinical need in Patients with BCCNS Vismodegib and Sonidegib FDA-Approved - Limited to Locally Advanced and/or Metastatic BCC - Response rates (30% to 58% range) in advanced disease - Serious Side-Effects (Grade 2 – 4) limit even short-term use - Neither approved for BCCNS – No insurance coverage - 54% drop out rate – vismodegib clinical testing for BCCNS 7 © 2017 HedgePath Pharmaceuticals, Inc. All rights reserved
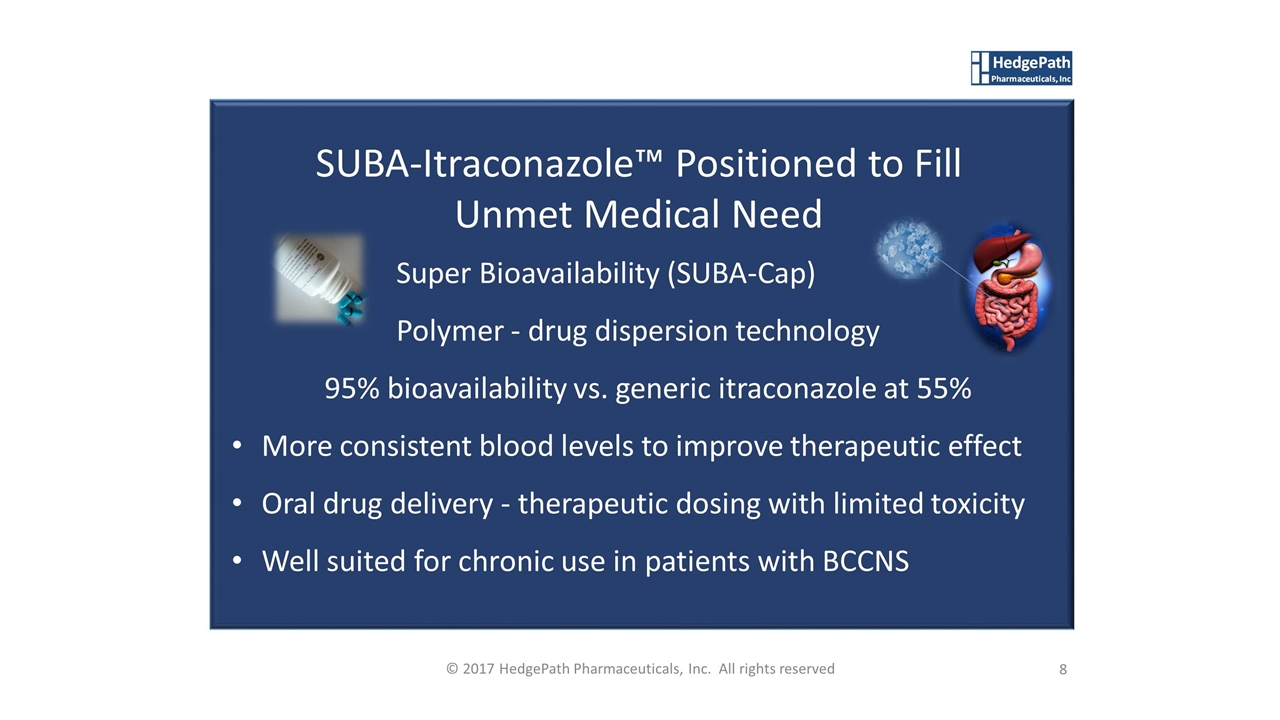
Super Bioavailability (SUBA-Cap) Polymer - drug dispersion technology 95% bioavailability vs. generic itraconazole at 55% More consistent blood levels to improve therapeutic effect Oral drug delivery - therapeutic dosing with limited toxicity Well suited for chronic use in patients with BCCNS SUBA-Itraconazole™ Positioned to Fill Unmet Medical Need 8 © 2017 HedgePath Pharmaceuticals, Inc. All rights reserved
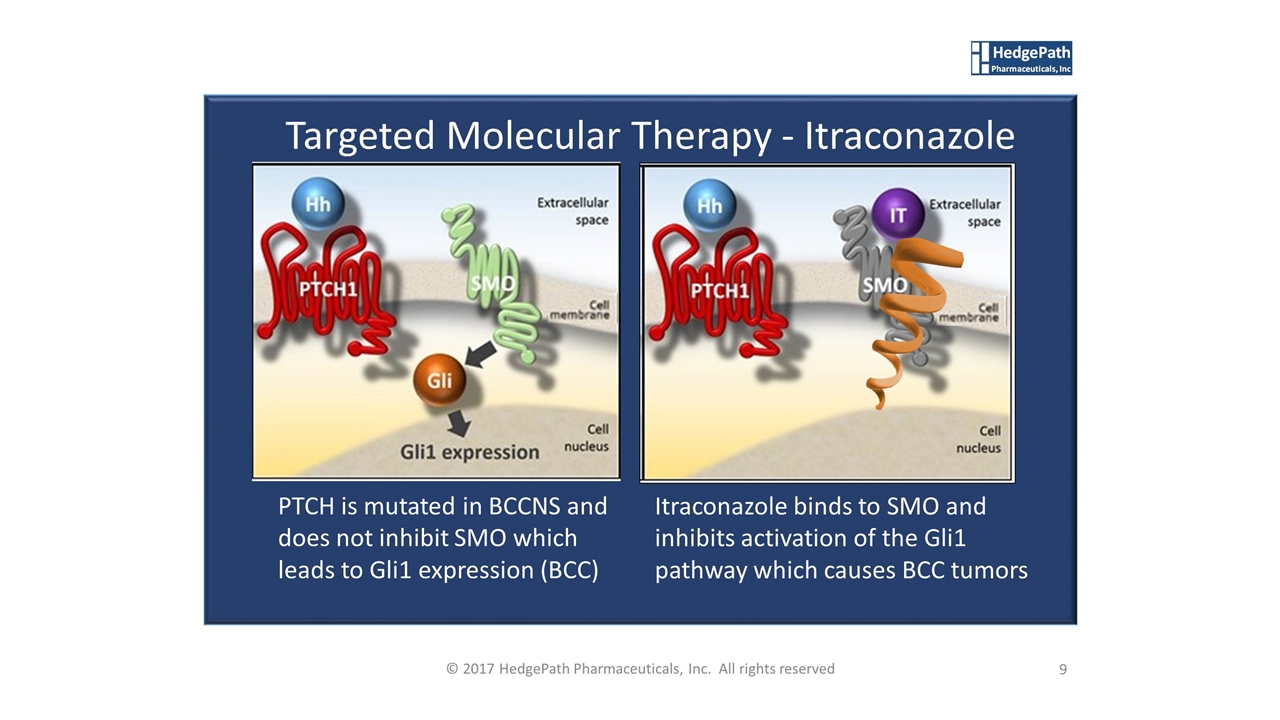
© 2017 HedgePath Pharmaceuticals, Inc. All rights reserved Targeted Molecular Therapy - Itraconazole PTCH is mutated in BCCNS and does not inhibit SMO which leads to Gli1 expression (BCC) Itraconazole binds to SMO and inhibits activation of the Gli1 pathway which causes BCC tumors 9
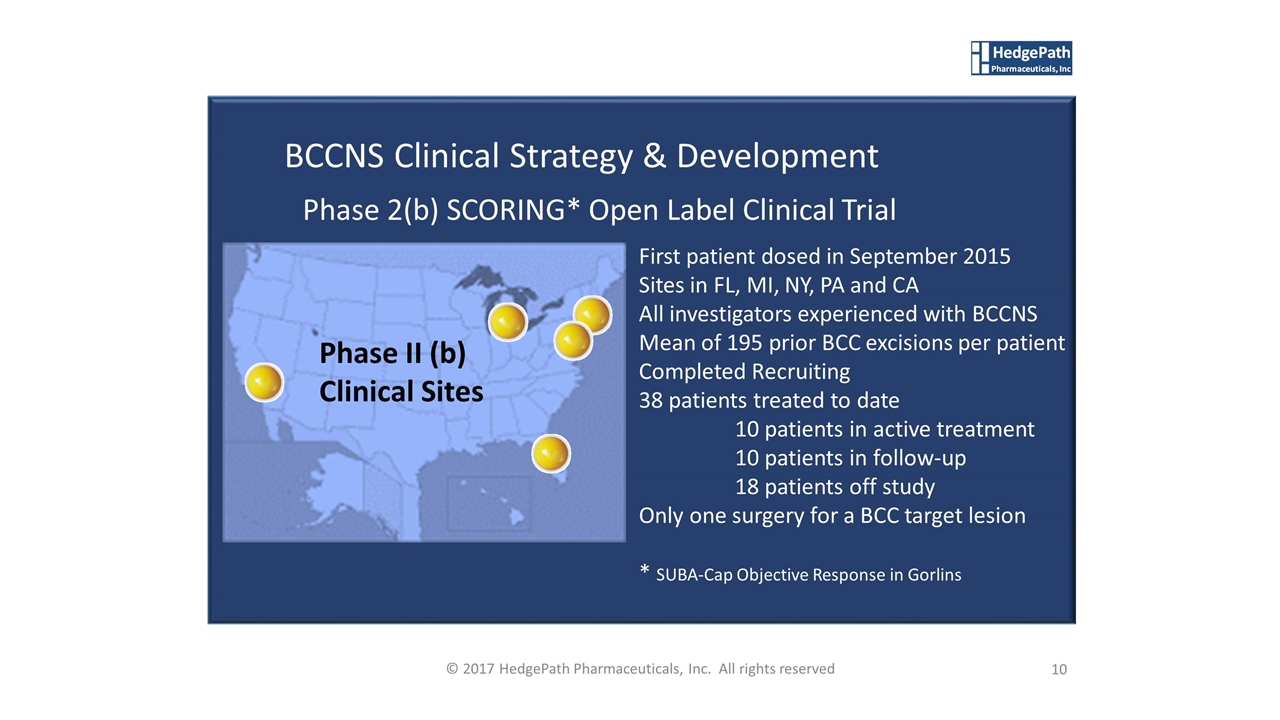
BCCNS Clinical Strategy & Development Phase 2(b) SCORING* Open Label Clinical Trial Phase II (b) Clinical Sites 10 © 2017 HedgePath Pharmaceuticals, Inc. All rights reserved First patient dosed in September 2015 Sites in FL, MI, NY, PA and CA All investigators experienced with BCCNS Mean of 195 prior BCC excisions per patient Completed Recruiting 38 patients treated to date 10 patients in active treatment 10 patients in follow-up 18 patients off study Only one surgery for a BCC target lesion * SUBA-Cap Objective Response in Gorlins
FDA Guidance from Type C Meeting A single-arm open label trial can lead to approval If the BCC tumor responses are robust If there is a meaningful duration of response If tumor responses occur in serious lesions that predict for clinical benefit, such as the delay or elimination of disfiguring surgeries “FDA agrees that RECIST may not be optimal for BCCNS” 11 © 2017 HedgePath Pharmaceuticals, Inc. All rights reserved
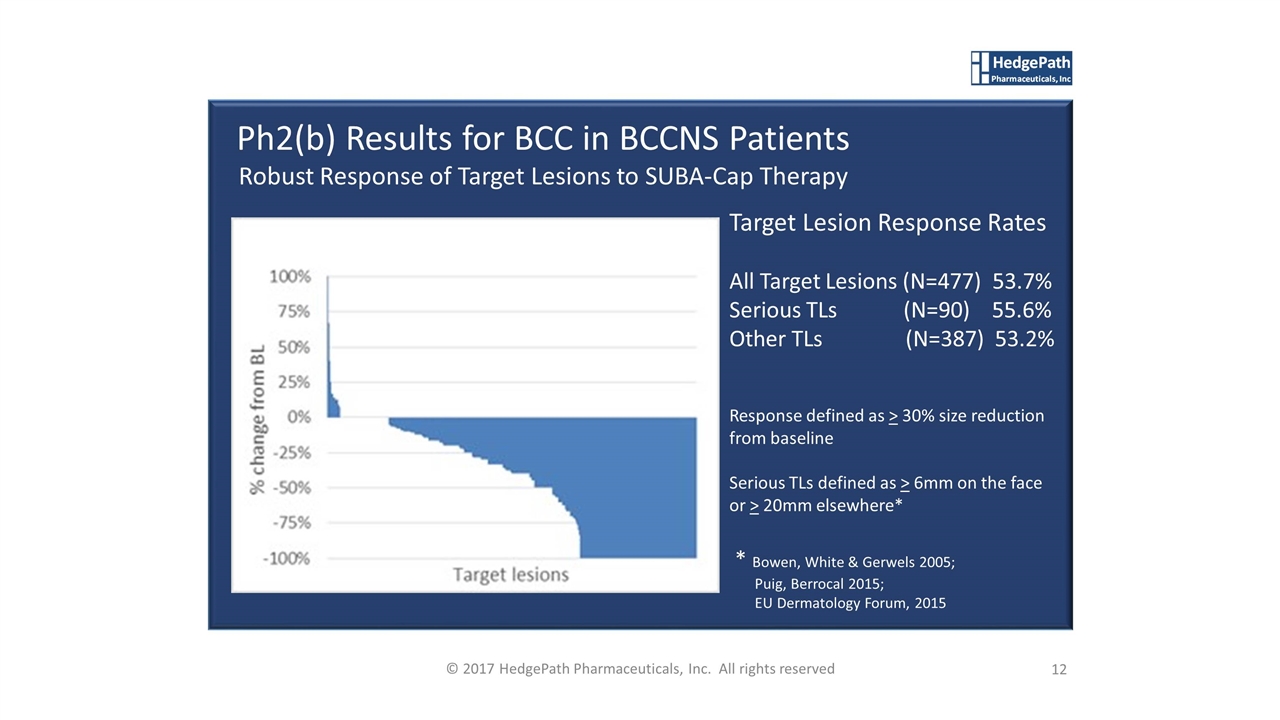
12 © 2017 HedgePath Pharmaceuticals, Inc. All rights reserved Ph2(b) Results for BCC in BCCNS Patients Robust Response of Target Lesions to SUBA-Cap Therapy Target Lesion Response Rates All Target Lesions (N=477) 53.7% Serious TLs (N=90) 55.6% Other TLs (N=387) 53.2% Response defined as > 30% size reduction from baseline Serious TLs defined as > 6mm on the face or > 20mm elsewhere* * Bowen, White & Gerwels 2005; Puig, Berrocal 2015; EU Dermatology Forum, 2015
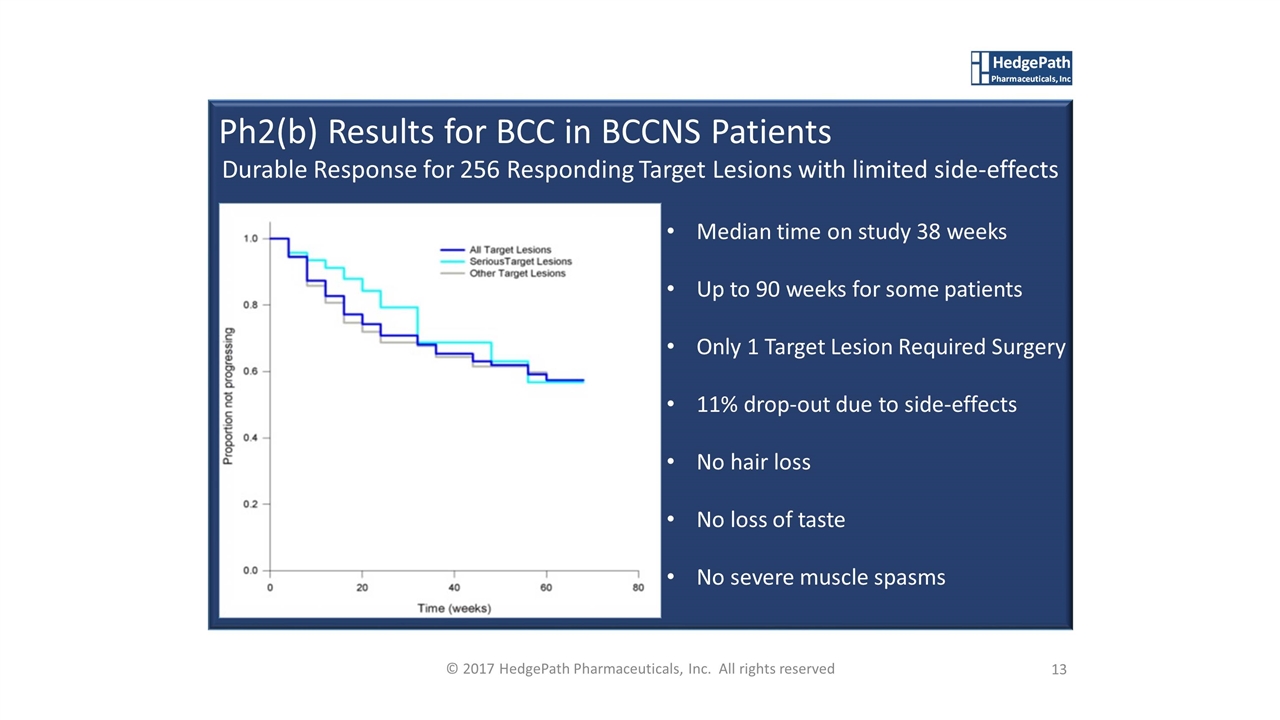
13 © 2017 HedgePath Pharmaceuticals, Inc. All rights reserved Median time on study 38 weeks Up to 90 weeks for some patients Only 1 Target Lesion Required Surgery 11% drop-out due to side-effects No hair loss No loss of taste No severe muscle spasms Ph2(b) Results for BCC in BCCNS Patients Durable Response for 256 Responding Target Lesions with limited side-effects
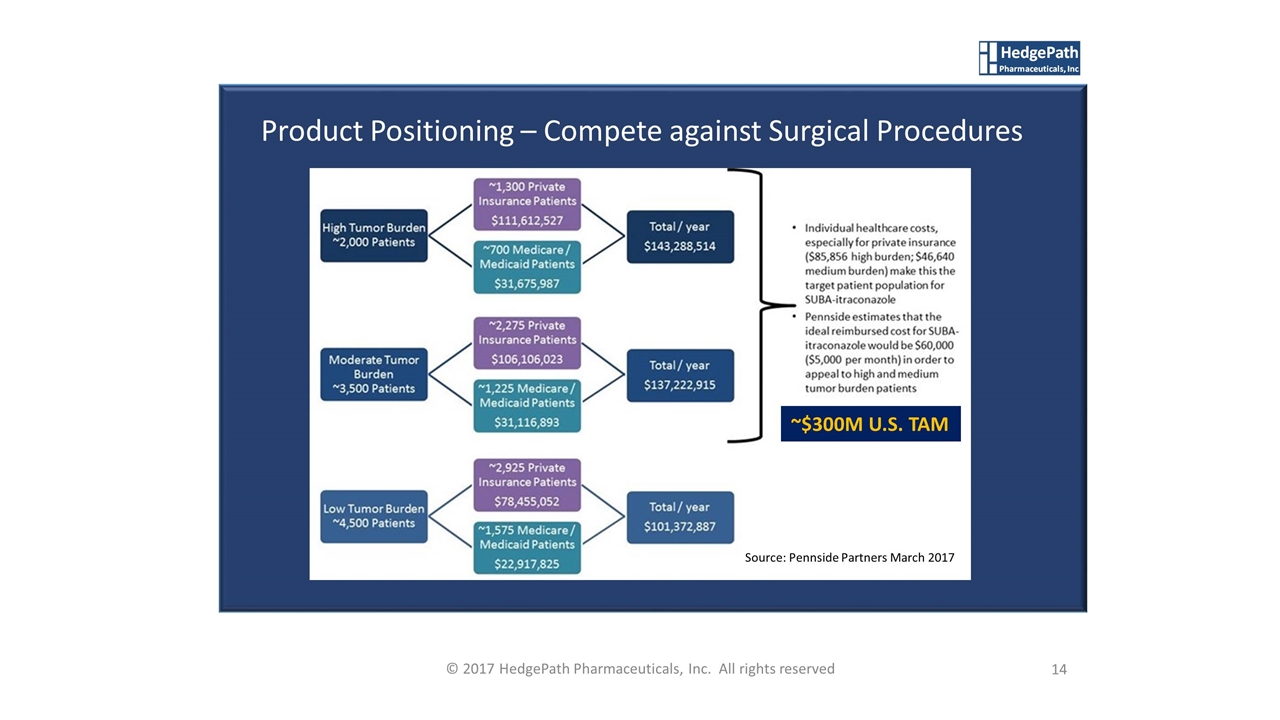
14 © 2017 HedgePath Pharmaceuticals, Inc. All rights reserved Product Positioning – Compete against Surgical Procedures Source: Pennside Partners March 2017 ~$300M U.S. TAM
SUBA-Cap Regulatory Strategy 505(b)(2) Regulatory Pathway – repurposing FDA-approved itraconazole Q4 2017 Individual Patient Data Review, Analysis & Follow-up Q1 2018 Database Lock and Clinical Study Report Q2 2018 Pre-NDA FDA Meeting Request Orphan Designation for BCCNS: - FDA recognizes that BCCNS is not a subset of Sporadic BCC - 7 years market exclusivity post-approval - 50% tax credit on cost of US clinical trials 15 © 2017 HedgePath Pharmaceuticals, Inc. All rights reserved

Phase 2 Physician-Sponsored JHU Trial Median Survival: improved from 8 months to 32 months in Phase II Physician-Sponsored Trial* Lung Cancer Opportunity 85% of lung cancers are NSCLC (non small cell lung cancer) SUBA-Itraconazole oral therapy may extend survival in late-stage disease Target: 58,000 Stage IV Non-Squamous NSCLC patients in the U.S. Goal: demonstrate efficacy in double-blind placebo controlled clinical trial Currently conducting Key Opinion Leader study on potential for SUBA-Cap in NSCLC * Journal of Thoracic Oncology, May 2013 -Johns Hopkins University, Rudin, et. al 16 © 2017 HedgePath Pharmaceuticals, Inc. All rights reserved

ADT (androgen deprivation therapy) has no evidence of clinical benefit in men who have castrate-resistant non-metastatic prostate cancer Itraconazole does not lower testosterone levels causing loss of libido Target: 190,000 men who have castrate-resistant non-metastatic PC in the U.S. Goal: Demonstrate efficacy for delaying time to bone metastases in men with NMCRPC in a double-blind placebo controlled trial Currently conducting Key Opinion Leader study on potential for SUBA-Cap in PC 90% of men with advanced disease in the Phase II Physician-Sponsored Trial who achieved therapeutic levels of itraconazole had dramatic reductions in PSA progression* * Johns Hopkins University, Antonarakis, et. al, The Oncologist, February 2013 17 © 2017 HedgePath Pharmaceuticals, Inc. All rights reserved Prostate Cancer Opportunity

HedgePath Pharmaceuticals, Inc. www.hedgepathpharma.com Nicholas J. Virca, President and CEO nvirca@hedgepathpharma.com 18 © 2017 HedgePath Pharmaceuticals, Inc. All rights reserved