EX-99.2
Published on August 20, 2019

Corporate Presentation August 2019 OTCQB:INTI Exhibit 99.2

Cautionary Note on Forward-Looking Statements and Disclaimers This presentation includes or incorporates by reference statements that constitute “forward-looking statements” within the meaning of the U.S. federal securities laws. These statements relate to future events or to our future performance, and involve significant known and unknown risks, uncertainties and other factors that may cause our actual results, levels of activity, performance, or achievements to be materially different from any future results, levels of activity, performance or achievements expressed or implied in these forward-looking statements. These statements include, but are not limited to, information or assumptions about our clinical development programs, our expenses, capital and other expenditures, our financing needs and plans, our capital structure, and management’s plans, goals and objectives for future operations and growth. In some cases, you can identify forward-looking statements by the use of words such as “may,” “could,” “expect,” “intend,” “plan,” “seek,” “anticipate,” “believe,” “estimate,” “predict,” “continue,” “or the negative of these terms or other comparable terminology. You should not place undue reliance on forward-looking statement since they involve known and unknown risks, uncertainties and other factors which are, in some cases, beyond our control and which could cause actual performance or results to differ materially from those expressed in or suggested by forward-looking statements. Important factors that could cause such differences include, but are not limited to: (i) risks and uncertainties associated with our research and development activities, including our anticipated clinical trials; (ii) our dependence on Mayne Pharma for the supply of our product candidate and key intellectual property; (iii) our ability to raise capital when needed; (iv) the timing of and our ability to achieve U.S. or international regulatory approvals for our product candidates; (v) our dependence on others to conduct clinical of, and to manufacture and market, our product candidates; (vi) our ability to gain market acceptance for our product candidates; (vii) our ability to maintain or protect the validity of patents and other intellectual property; (viii) our ability to secure registration for our current and future patent applications; and (ix) our ability to attract and retain key personnel. Should one or more of these risks or uncertainties materialize, or should any of our assumptions prove incorrect, actual results may vary in material or even significant respects from those projected in these forward-looking statements. We do not undertake any obligation to update or revise any forward-looking statements, whether as a result of new information, future events or otherwise, except as may be required under applicable securities laws. Peak sales estimates have been determined on the basis of market research and comparable product analysis, but no assurances can be given that such sales levels will be achieved, if at all. This presentation does not constitute an offer or any securities for sale or solicitation of an offer to buy any securities, nor shall there be any sale of the securities in any state or jurisdiction in which such an offer, solicitation or sale would be unlawful prior to the registration or qualification under the securities laws of any such state or jurisdiction.
Mission Statement We are focused on the discovery, development and commercialization of innovative therapeutics to inhibit the progression of cancerous and non-cancerous proliferation disorders Skin Cancer Itraconazole- based Therapies Prostate Cancer Orphan Oncology Proliferation Disorders Lung Cancer
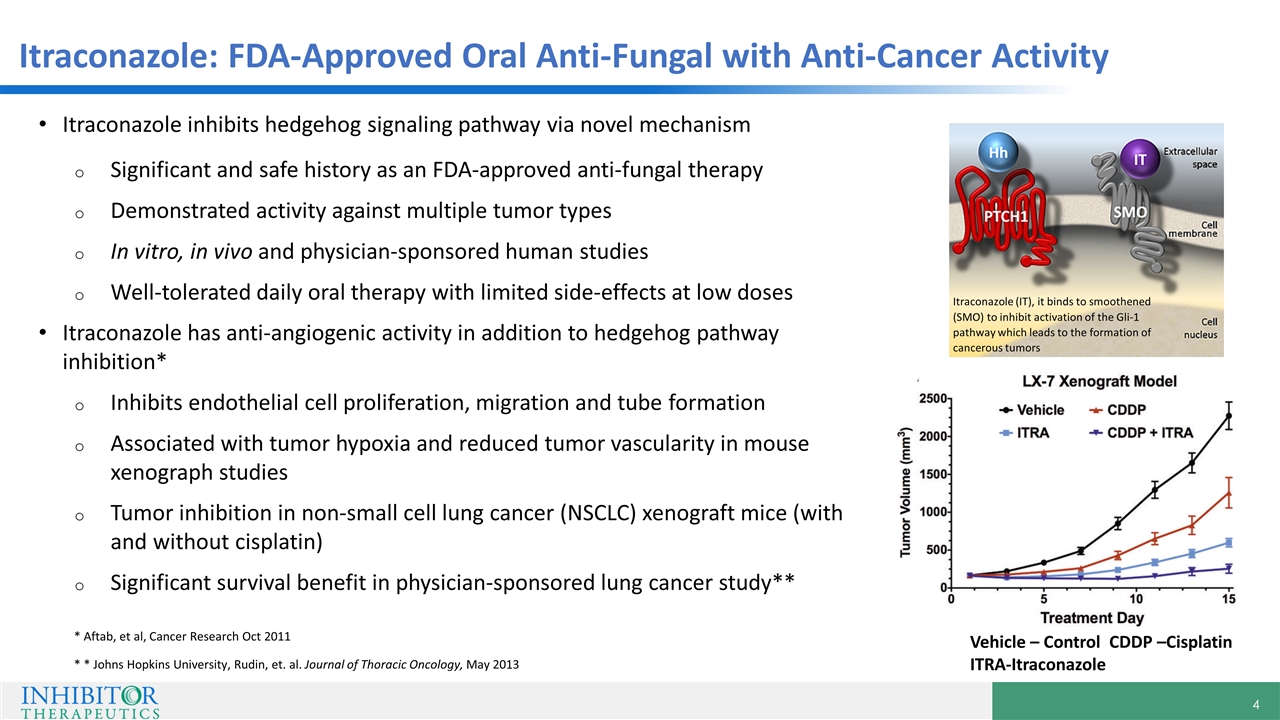
Itraconazole: FDA-Approved Oral Anti-Fungal with Anti-Cancer Activity Itraconazole inhibits hedgehog signaling pathway via novel mechanism Significant and safe history as an FDA-approved anti-fungal therapy Demonstrated activity against multiple tumor types In vitro, in vivo and physician-sponsored human studies Well-tolerated daily oral therapy with limited side-effects at low doses Itraconazole has anti-angiogenic activity in addition to hedgehog pathway inhibition* Inhibits endothelial cell proliferation, migration and tube formation Associated with tumor hypoxia and reduced tumor vascularity in mouse xenograph studies Tumor inhibition in non-small cell lung cancer (NSCLC) xenograft mice (with and without cisplatin) Significant survival benefit in physician-sponsored lung cancer study** * Aftab, et al, Cancer Research Oct 2011 * * Johns Hopkins University, Rudin, et. al. Journal of Thoracic Oncology, May 2013 Vehicle – Control CDDP –Cisplatin ITRA-Itraconazole Itraconazole (IT), it binds to smoothened (SMO) to inhibit activation of the Gli-1 pathway which leads to the formation of cancerous tumors
Challenges with Generic Itraconazole as an Anti-Cancer Therapy Itraconazole is poorly bioavailable (~55% when dosed orally) Inconsistent blood plasma levels from one dose to the next Inconsistent blood plasma levels between patients at the same dose Less predictable at higher doses required for anti-cancer therapies Requires meal intake plus the addition of acidic beverages Contraindicated for patients with achlorhydria (low acid stomach) Not to be used with proton pump inhibitors prescribed for acid reflux
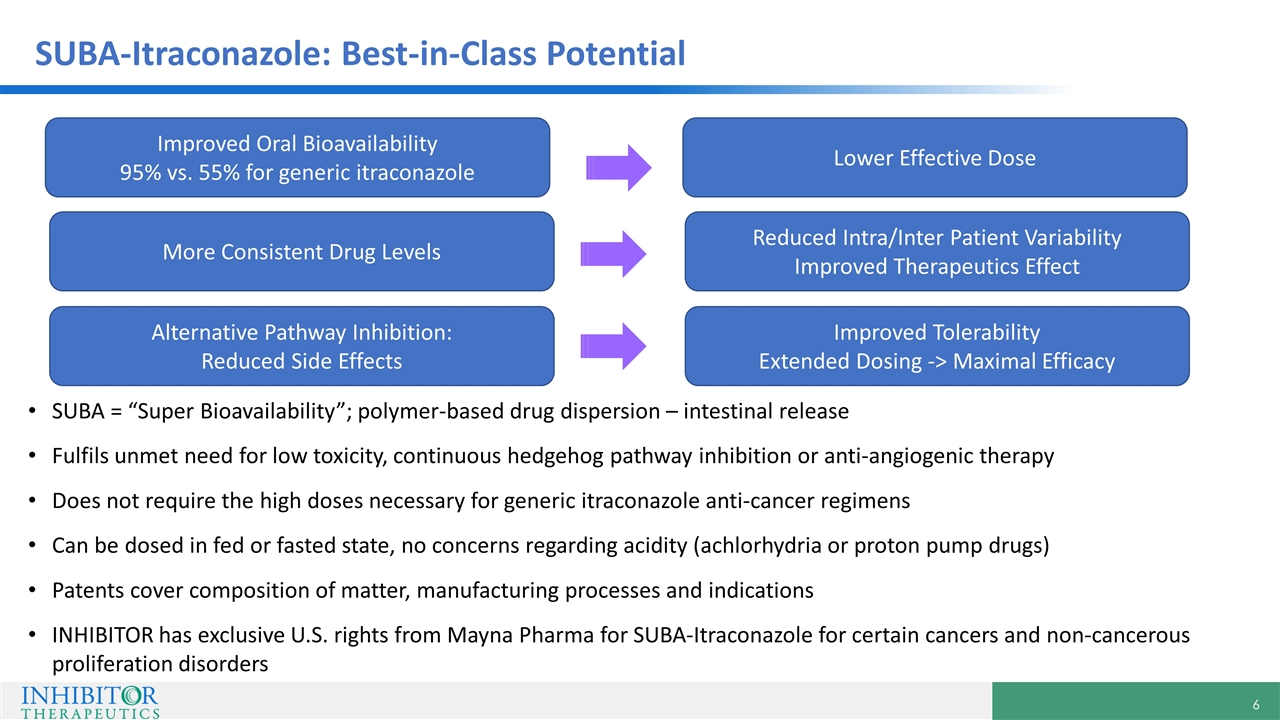
SUBA-Itraconazole: Best-in-Class Potential SUBA = “Super Bioavailability”; polymer-based drug dispersion – intestinal release Fulfils unmet need for low toxicity, continuous hedgehog pathway inhibition or anti-angiogenic therapy Does not require the high doses necessary for generic itraconazole anti-cancer regimens Can be dosed in fed or fasted state, no concerns regarding acidity (achlorhydria or proton pump drugs) Patents cover composition of matter, manufacturing processes and indications INHIBITOR has exclusive U.S. rights from Mayna Pharma for SUBA-Itraconazole for certain cancers and non-cancerous proliferation disorders Improved Oral Bioavailability 95% vs. 55% for generic itraconazole More Consistent Drug Levels Alternative Pathway Inhibition: Reduced Side Effects Lower Effective Dose Reduced Intra/Inter Patient Variability Improved Therapeutics Effect Improved Tolerability Extended Dosing -> Maximal Efficacy
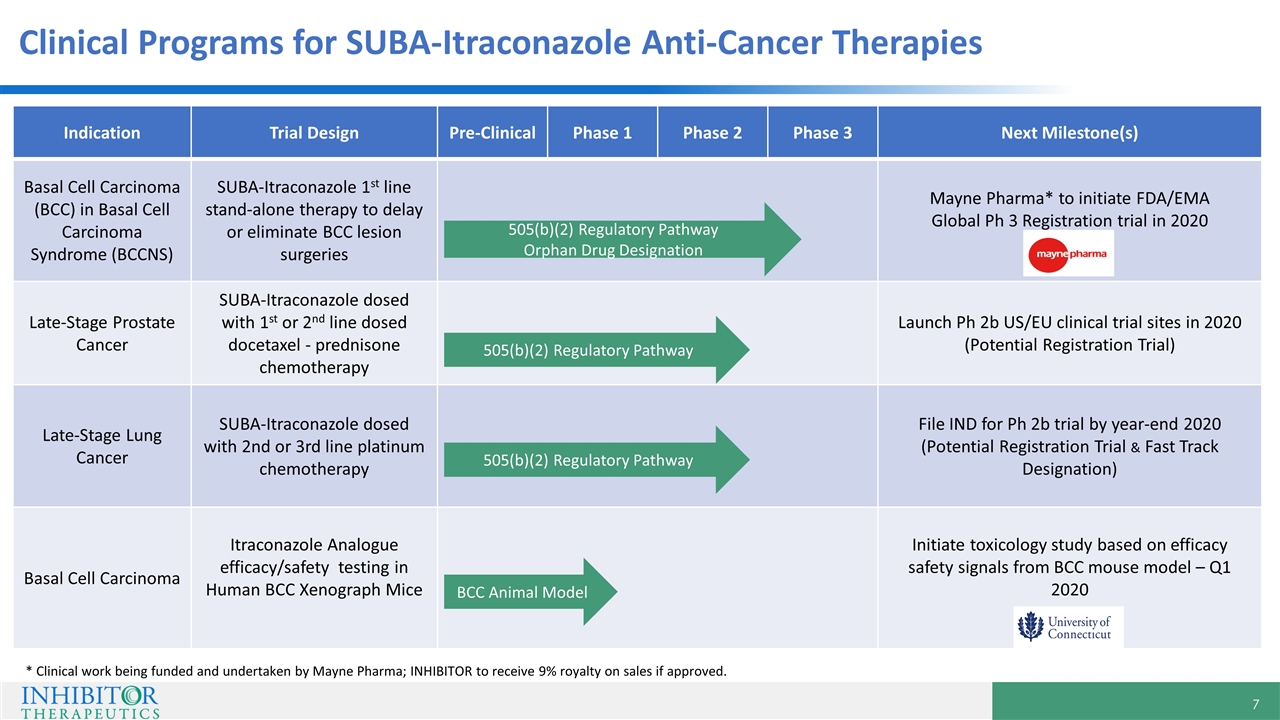
Indication Trial Design Pre-Clinical Phase 1 Phase 2 Phase 3 Next Milestone(s) Basal Cell Carcinoma (BCC) in Basal Cell Carcinoma Syndrome (BCCNS) SUBA-Itraconazole 1st line stand-alone therapy to delay or eliminate BCC lesion surgeries Mayne Pharma* to initiate FDA/EMA Global Ph 3 Registration trial in 2020 Late-Stage Prostate Cancer SUBA-Itraconazole dosed with 1st or 2nd line dosed docetaxel - prednisone chemotherapy Launch Ph 2b US/EU clinical trial sites in 2020 (Potential Registration Trial) Late-Stage Lung Cancer SUBA-Itraconazole dosed with 2nd or 3rd line platinum chemotherapy File IND for Ph 2b trial by year-end 2020 (Potential Registration Trial & Fast Track Designation) Basal Cell Carcinoma Itraconazole Analogue efficacy/safety testing in Human BCC Xenograph Mice Initiate toxicology study based on efficacy safety signals from BCC mouse model – Q1 2020 505(b)(2) Regulatory Pathway 505(b)(2) Regulatory Pathway 505(b)(2) Regulatory Pathway Orphan Drug Designation Clinical Programs for SUBA-Itraconazole Anti-Cancer Therapies BCC Animal Model * Clinical work being funded and undertaken by Mayne Pharma; INHIBITOR to receive 9% royalty on sales if approved.
Clinical Experience Supports Pipeline Expansion Completed Ph 2b trial in Basal Cell Carcinoma Nevus Syndrome (BCCNS) in 38 patients across 5 U.S. sites SUBA-Itraconazole decreased 57% of 477 BCC target lesions by 30% or more in size Only 6 lesions required surgical excision across all 38 patients 130 BCC target lesions (27%) completely disappeared Demonstrated an improved toxicity profile (11% drop-out vs. 54% for vismodegib in Genentech BCCNS study) INHIBITOR management and clinical/regulatory teams demonstrated solid execution of first BCCNS trial Confirmed 505(b)(2) regulatory pathway and awarded Orphan Drug Designation for BCCNS Mayne Pharma preparing to initiate Global Ph 3 trial in BCCNS based on INHIBITOR Ph 2b study results Mayne pursuing regulatory guidance (FDA and EMA) to specify study endpoints for marketing approval Physician-sponsored trials also demonstrated proof of concept for itraconazole use in prostate and lung cancers INHIBITOR will pursue 505(b)(2) pathway for SUBA-Itraconazole use with chemotherapy in those indications
Opportunity for SUBA-Itraconazole in Late-Stage Prostate Cancer Metastatic, Castrate-Resistant Prostate Cancer (mCRPC) Aberrant hedgehog signaling suggests a direct role in prostate cancer* Promotes tumor formation and invasion Contributes toward development of castrate-resistance and ADT failure Second most common cause of cancer-related deaths in the U.S. ~30,000 men per year First-line local and systemic therapies include surgery, radiation, and androgen deprivation therapy (ADT) All patients with late-stage disease eventually become refractory to hormonal therapy (castrate-resistant) >23,000 Men (U.S.) * Sing, et al, American Journal of Pathology 2014 , Susman, Antonarakis, Sep 2015 Cancers MDPI
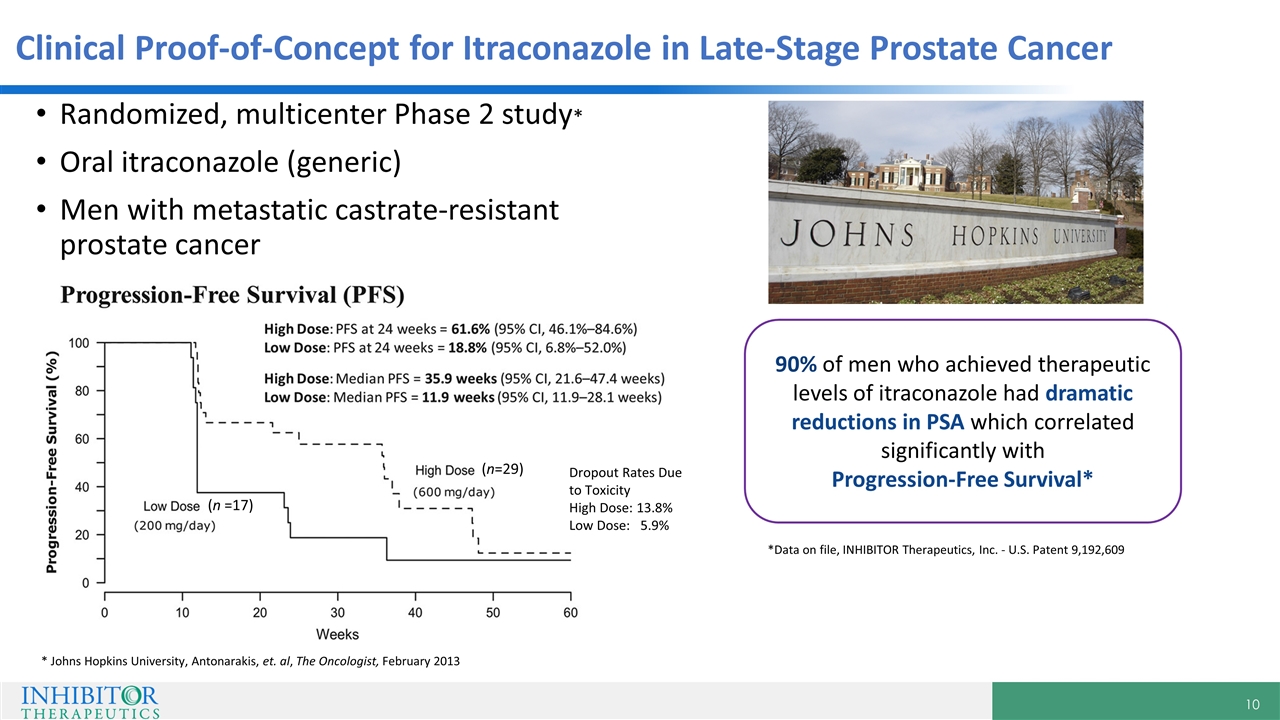
Clinical Proof-of-Concept for Itraconazole in Late-Stage Prostate Cancer Randomized, multicenter Phase 2 study* Oral itraconazole (generic) Men with metastatic castrate-resistant prostate cancer 90% of men who achieved therapeutic levels of itraconazole had dramatic reductions in PSA which correlated significantly with Progression-Free Survival* *Data on file, INHIBITOR Therapeutics, Inc. - U.S. Patent 9,192,609 * Johns Hopkins University, Antonarakis, et. al, The Oncologist, February 2013 (n =17) (n=29) Dropout Rates Due to Toxicity High Dose: 13.8% Low Dose: 5.9%
Clinical Strategy for SUBA-Itraconazole in Late-Stage Prostate Cancer Phase 2b PREDICT* Trial Design: Randomized, controlled, multi-center clinical trial Oral SUBA-Itraconazole in combination with standard of care docetaxel-prednisone chemotherapy Objective: Evaluate the ability to delay disease progression in high-risk men with metastatic castration-resistant prostate cancer (mCRPC) April 2019 : Face-to-Face Pre-IND FDA Meeting Agreements Primary endpoint of rPFS (radiographic progression-free survival) Potential for registration based on End of Phase 2 (EOP2) Protocol and Statistical Analysis Plan Allowed to leverage itraconazole and SUBA-Itraconazole safety data Confirmed 505(b)(2) regulatory pathway; Right of reference to Mayne Pharma Drug Master File 2019 Upcoming Milestones Q4: EoP2 Meeting (potential FDA signoff on registrational design); IND clearance to proceed with Ph 2b multi-center, double-blind, randomized placebo-controlled trial * Prostate Response Evaluating Docetaxel Itraconazole Combination Therapy
Opportunity for SUBA-Itraconazole in Late-Stage Lung Cancer Significant unmet need for patients with late-stage disease requiring chemotherapy Lung cancer is the leading cause of cancer-related deaths in the U.S.: 160,000 / year > colon, breast & prostate combined 85% are non-small cell lung cancer (NSCLC) ~75,000 newly diagnosed at Stage 4 / year Itraconazole has anti-angiogenic mechanisms in addition to hedgehog pathway inhibition Inhibits endothelial cell proliferation, migration, and tube formation Associated with tumor hypoxia and reduced tumor vascularity in mouse xenograft studies * Johns Hopkins University, Rudin, et. al. Journal of Thoracic Oncology, May 2013; American Cancer Society 2018 Only ~50% of patients with advanced, non-squamous NSCLC are candidates for targeted chemotherapy drugs or immunotherapy* ~45,000 patients per year (U.S.)
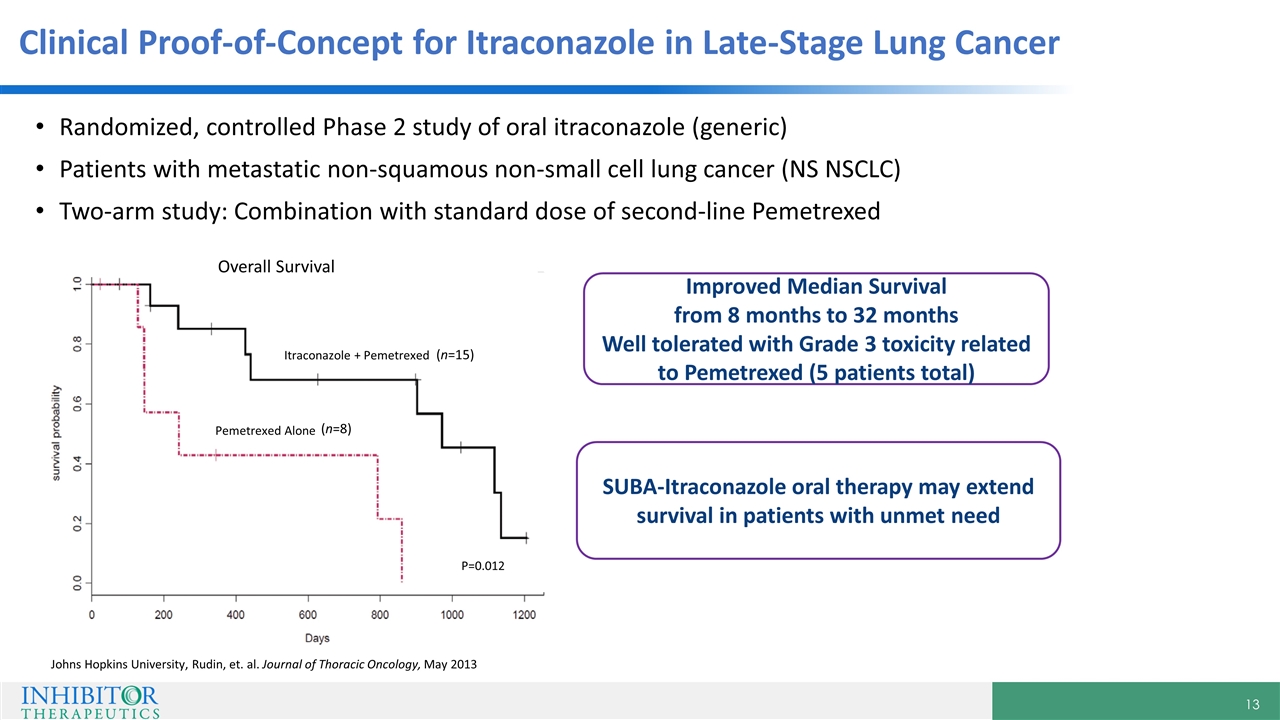
Randomized, controlled Phase 2 study of oral itraconazole (generic) Patients with metastatic non-squamous non-small cell lung cancer (NS NSCLC) Two-arm study: Combination with standard dose of second-line Pemetrexed Overall Survival Itraconazole + Pemetrexed Pemetrexed Alone P=0.012 Improved Median Survival from 8 months to 32 months Well tolerated with Grade 3 toxicity related to Pemetrexed (5 patients total) SUBA-Itraconazole oral therapy may extend survival in patients with unmet need Johns Hopkins University, Rudin, et. al. Journal of Thoracic Oncology, May 2013 Clinical Proof-of-Concept for Itraconazole in Late-Stage Lung Cancer (n=15) (n=8)
Clinical Strategy for SUBA-Itraconazole in Late-Stage Lung Cancer Phase 2b Trial Design: Randomized, Controlled, Multi-Center Oral SUBA-Itraconazole in combination with standard of care chemotherapy Objective: Evaluate the ability to delay disease progression in patients with advanced non-squamous non-small cell lung cancer Upcoming Milestones 2020: IND clearance for Phase 2b study via 505(b)(2) pathway in NS NSCLC Pursue FDA Fast Track and/or Breakthrough Therapy Designation status based on progression-free survival benefit in patients with late-stage NS NSCLC
Preclinical Strategy for Itraconazole Analogues (IAs)* in Basal Cell Carcinoma (BCC) Pre-clinical Trial Design: Basal Cell Carcinoma Mouse Model Oral IA dosing (gavage fed mouse model with human BCC xenographs) Objective: Evaluate the efficacy and safety of IA against human BCC Upcoming Milestones Q4 2019: Evaluate BCC mouse model efficacy and safety for IA dosing Q1 2020: Initiate toxicology studies assuming efficacy signals in human BCC mouse study * Itraconazole Analogues (IAs) are subject to a worldwide exclusive option agreement with The University of Connecticut
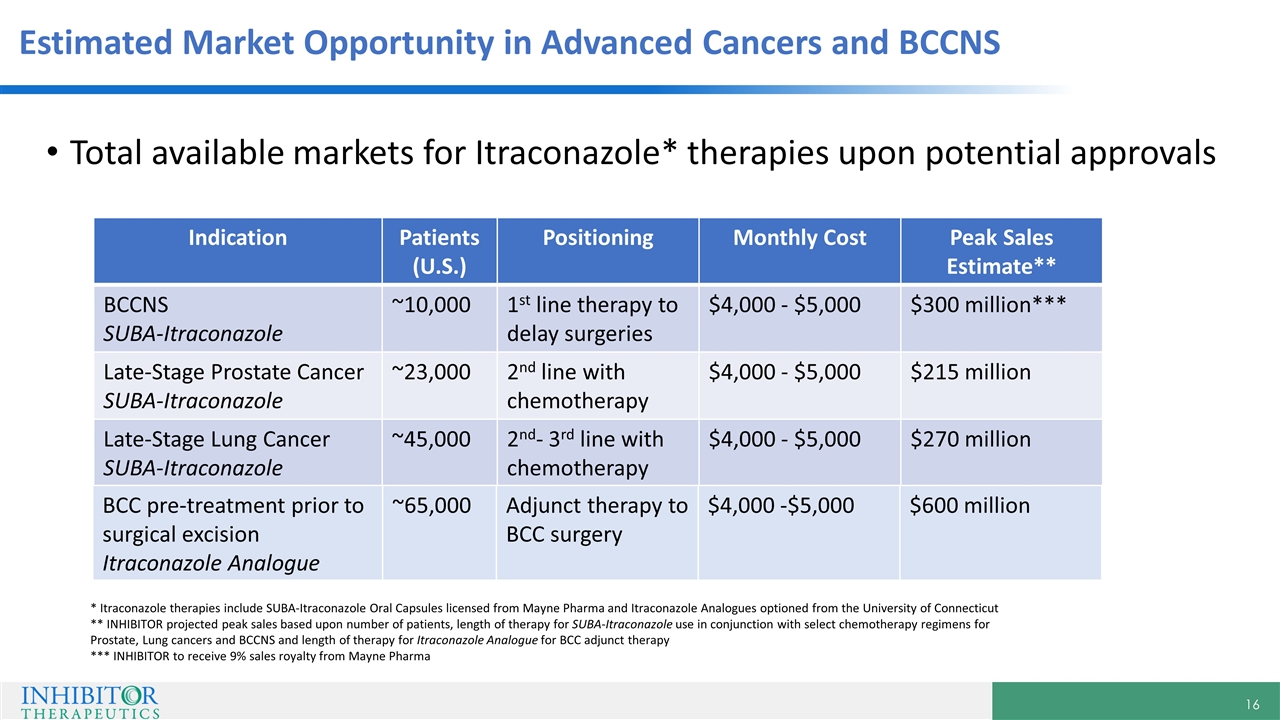
Estimated Market Opportunity in Advanced Cancers and BCCNS Total available markets for Itraconazole* therapies upon potential approvals * Itraconazole therapies include SUBA-Itraconazole Oral Capsules licensed from Mayne Pharma and Itraconazole Analogues optioned from the University of Connecticut ** INHIBITOR projected peak sales based upon number of patients, length of therapy for SUBA-Itraconazole use in conjunction with select chemotherapy regimens for Prostate, Lung cancers and BCCNS and length of therapy for Itraconazole Analogue for BCC adjunct therapy *** INHIBITOR to receive 9% sales royalty from Mayne Pharma Indication Patients (U.S.) Positioning Monthly Cost Peak Sales Estimate** BCCNS SUBA-Itraconazole ~10,000 1st line therapy to delay surgeries $4,000 - $5,000 $300 million*** Late-Stage Prostate Cancer SUBA-Itraconazole ~23,000 2nd line with chemotherapy $4,000 - $5,000 $215 million Late-Stage Lung Cancer SUBA-Itraconazole ~45,000 2nd- 3rd line with chemotherapy $4,000 - $5,000 $270 million BCC pre-treatment prior to surgical excision Itraconazole Analogue ~65,000 Adjunct therapy to BCC surgery $4,000 -$5,000 $600 million
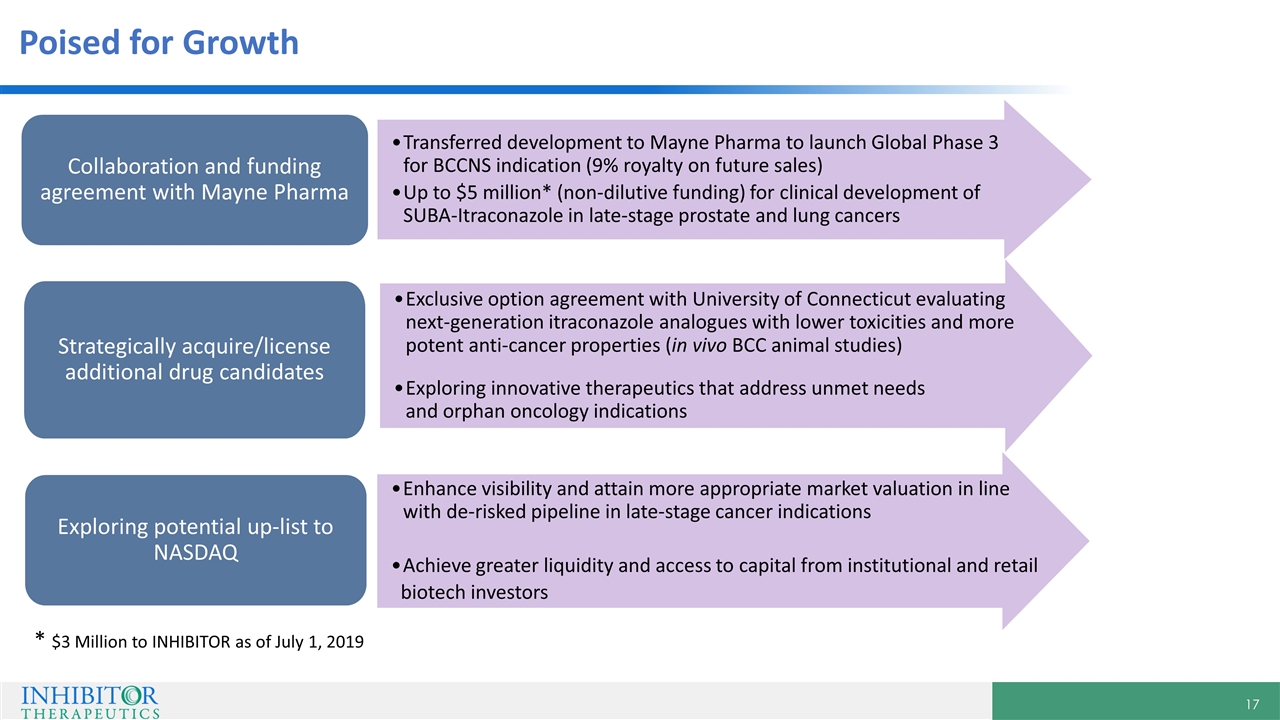
Poised for Growth Collaboration and funding agreement with Mayne Pharma Strategically acquire/license additional drug candidates Exploring potential up-list to NASDAQ Exclusive option agreement with University of Connecticut evaluating next-generation itraconazole analogues with lower toxicities and more potent anti-cancer properties (in vivo BCC animal studies) Exploring innovative therapeutics that address unmet needs and orphan oncology indications Transferred development to Mayne Pharma to launch Global Phase 3 for BCCNS indication (9% royalty on future sales) Up to $5 million* (non-dilutive funding) for clinical development of SUBA-Itraconazole in late-stage prostate and lung cancers Enhance visibility and attain more appropriate market valuation in line with de-risked pipeline in late-stage cancer indications Achieve greater liquidity and access to capital from institutional and retail biotech investors * $3 Million to INHIBITOR as of July 1, 2019

Management Team Nicholas Virca, President & Chief Executive Officer 30 years in Human Therapeutics and Diagnostics (20 years at C Level) Co-founder HedgePath, Adventrx Pharmaceuticals, Diametrix Detectors, and Damon Biotech Garrison Hasara, CPA, Chief Financial Officer 20 years in Accounting and Finance (including biotech PIPEs and S1 – S3 financings) Previous senior management positions in finance with Accentia Pharmaceuticals, Automotive Service Centers, and Kforce Amy McCord, Ph.D., RAC, Vice President, Regulatory Affairs * 10 years Regulatory & Scientific Research (Ph.D. in Biomedical Sciences-USF) Previous senior management positions with DSI, Inc., Cardinal Health, Biovest International, Accentia Biopharmaceuticals, and Moffitt Cancer Center USF *Consultant contact

Board of Directors Mark Watson, CPA - Chairman 40 years in Public Accounting and Auditing in healthcare and life sciences Retired Deloitte Touche Tohmatsu, serves on the boards of Sykes Enterprise and Biotechnology Sciences International Member of American Institute of Public Accountants Robert D. Martin - Director 30 years experience in Financial Consulting and Operations Previous experience with The Interlochen Group, Tandy Brands Accessories, Intezyne, and Russell Corporation Stefan J. Cross – Director 20 years experience in Pharmaceuticals Current and previous management positions with Mayne Pharma as President of US Subsidiary of Mayne, VP Business Development of non-US operations and former Head of Marketing Asia Pacific for Hospira Dana R. Ono, Ph.D. – Director 35 years C-level experience in Public and Private Life Science companies and Venture Capital Co-founder VIMAC Media Fund, founder of several biotech companies in drug discovery and development; founder of the Massachusetts Biotechnology Council and on the board of Marine Biological Laboratory Woods Hole, Massachusetts
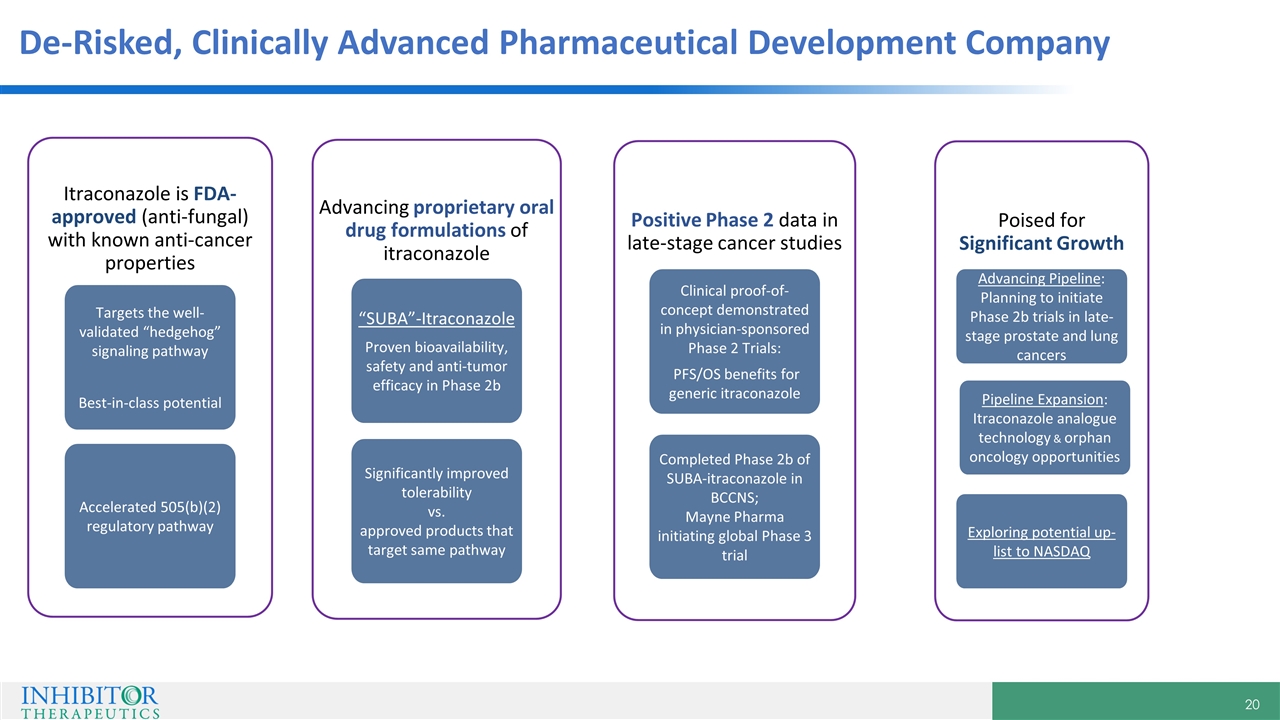
De-Risked, Clinically Advanced Pharmaceutical Development Company Positive Phase 2 data in late-stage cancer studies Clinical proof-of-concept demonstrated in physician-sponsored Phase 2 Trials: PFS/OS benefits for generic itraconazole Completed Phase 2b of SUBA-itraconazole in BCCNS; Mayne Pharma initiating global Phase 3 trial Itraconazole is FDA-approved (anti-fungal) with known anti-cancer properties Targets the well-validated “hedgehog” signaling pathway Best-in-class potential Accelerated 505(b)(2) regulatory pathway Poised for Significant Growth Advancing Pipeline: Planning to initiate Phase 2b trials in late-stage prostate and lung cancers Pipeline Expansion: Itraconazole analogue technology & orphan oncology opportunities Exploring potential up-list to NASDAQ Advancing proprietary oral drug formulations of itraconazole “SUBA”-Itraconazole Proven bioavailability, safety and anti-tumor efficacy in Phase 2b Significantly improved tolerability vs. approved products that target same pathway
Contact Us For Additional Information: Sharen Tilman Tiberend Strategic Advisors, Inc. 212-375-2664 office stilman@tiberend.com INHIBITOR Therapeutics, Inc. 4830 W. Kennedy Blvd. Suite 600 Tampa, Florida 33609